Organic compounds can basically be identified as compounds that contain carbon-hydrogen bonds. And while this is one distinguishing characteristic, there are many other ways in which organic compounds can be classified.
Get to know more about the different classes of organic compounds.
Compounds of Organic: Origin
Before Friedrich Wohler’s discovery in the early 1800s, organic chemistry was limited to studying substances produced naturally by living organisms. However, thanks to Wohler’s work, organic compounds can now be synthesised from minerals and other non-organic materials in the laboratory.
Carbon is the primary constituent of organic molecules, and it is nearly always linked to another carbon atom or a hydrogen atom as well. Because of carbon’s outstanding characteristics in synthetic compounds and insecticides, current chemical and materials sciences have focused on that element.
Phosphorus, nitrogen, and oxygen may all be found bonded to carbons on occasion. Some carbon compounds aren’t even regarded to be organic molecules, such as benzene and tin. The carbon compounds include carbon dioxide and monoxide, cyanates, cyanides, and various other carbon-based ions.
Carbon may also hold other elements, including phosphorus, nitrogen, and oxygen. Some carbon compounds aren’t considered organic because of their chemical structure. The carbon compounds include carbon dioxide and monoxide, cyanates, cyanides, and various other carbon-based ions.
Chemicals like ethanol and isopropanol are examples of alcohols, and they’re antiseptics, and ethanol is a common ingredient in beverages. Last but not least, carboxylic acids are present in a wide variety of substances, including drugs. In aspirin, one of the most well-known commercial drugs, carboxylic acid may be found.
Even though millions of organic molecules exist, even the most complicated ones may be classified using a basic system and named using a procedure. It will cover the classification of organic compounds in this course and some of the most common organic compounds.
What is the definition of an Organic Compound?
Carbon is a fundamental component of organic substances, as are other elements required for living creatures to reproduce. With four electrons and an outer shell that can hold eight electrons, carbon is the primary component.
It may form different bonds with other carbon atoms and elements like hydrogen, oxygen, and nitrogen—examples of organic compounds that may form lengthy chains and complicated structures are hydrocarbons and proteins.
Chemical reactions in plant and animal cells are based on the organic compounds made up of these molecules. These reactions supply the energy needed to obtain food, multiply, and perform all other life-related operations.
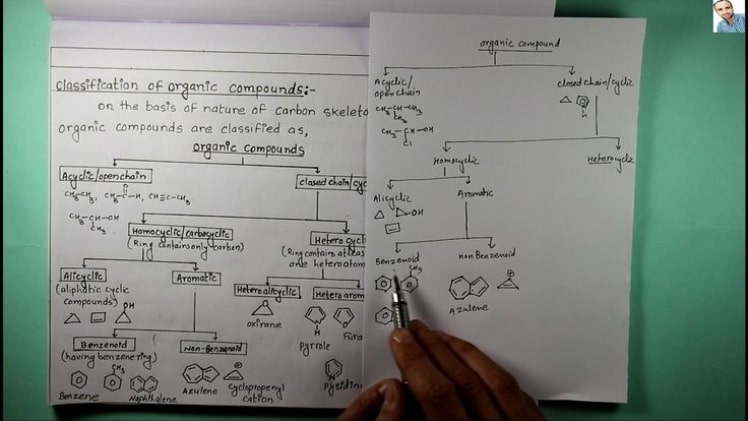
Classification of Organic Compounds
Lewis structures, space-filled models, and structural formulae are examples of organic compounds in various forms. Hydrogens are often seen as lines or left altogether in an organic molecule’s structural formula, and they’re thought to be there to finish off the 4-bonds the carbon atoms started.
Mass spectra have shown the presence of organic molecules. Results show that all extractants were capable of performing immediate analysis without a clean-up step after the extraction. However, the extracted organic compounds’ shape depended on the solvent, indicating their differential capacity to solubilise different biosolid organic compounds.
Following are the ways to classify Organic Compounds:
(1) Compounds with an open-chain carbon chain, such as acyclic or aliphatic compounds:
Acyclic or open-chain compounds have carbon atoms connected in a linear or branching form, resulting in an open-chain structure in the molecule. Aliphatic compounds are another name for these substances (Greek word: alpha meaning fat).
Examples:
Propane is composed of three chemical elements: CH3, CH3, and CH3.
(2) Compounds with a closed chain or a cyclic structure:
Closed chain compounds or ring compounds refer to organic compounds that have an atom chain that is closed.
There are many subtypes of these chemicals.
-
Homocyclic or carbocyclic compounds:
Compounds with solely carbon atoms in the ring structure are known as homocyclic compounds. There are many subtypes of these chemicals. - Aromatic Compounds:
Compounds classed as aromatic benzenoid compounds have one or more aromatic rings in their structure. The majority of these substances have a pleasant fragrance (Greek: Aroma – sweet smell).
-
Alicyclic compounds:
Also known as carbocyclic compounds, they are cyclic compounds with just carbon atoms in the ring structure. Despite their ring shape, these molecules act more like aliphatic ones.
-
Heterocyclic compounds (Non – benzenoid aromatic):
In addition to carbon atoms, heterocyclic compounds include cyclic compounds in which the ring atoms are made up of heteroatoms such as nitrogen, oxygen, and sulfur.
Characteristics of Organic Compounds
All organic compounds share the following qualities
- A large number of organic molecules may catch fire.
- The vast majority of the substances are covalent.
- Non-polar solvents like carbon tetrachloride, benzene, and others dissolve them quickly.
- Low melting and boiling points.
- Isomerism is a characteristic of this group.
- It may form families and homologous series of organic compounds based on the functional groups of the substances.
Classification of Organic Compounds Based on Characteristics
1. Functional group
Organic molecules’ distinctive chemical characteristics are partly due to the functional groups, which are made up of atoms or groups linked together uniquely. Examples are the hydroxyl group -OH, the aldehyde group -CHO, and the carboxylic acid group -COOH.
2. Homologous series
A homologous series is a collection of organic compounds, each of which has the same distinctive functional group but varies from the others by a single fixed unit. It’s possible to describe the homologous series members using a generic formula, and the succeeding members are distinguished from one another in the molecular formula by an additional CH2 unit.
For example, inorganic chemistry has a slew of homologous series like alkanes, alkenes, and alkynes.
Example of an organic compound
Gasoline is fuel, plastics are a type of detergent, and colourants are fuel. Even though both soap and detergent are used for washing, they are examples of two different types of organic chemistry.
Is there any purpose for organic compounds?
Because all living creatures include carbon, organic substances are essential. Many of the cycles in the world’s history are propelled by these fundamental elements. Consider the carbon cycle, in which carbon is exchanged between plants and animals during photosynthesis and cell respiration.
Conclusion
Because there are so many chemical components, it was necessary to organise them systematically. Acyclic (open chain) and cyclic organic molecules are the two main types of organic compounds (closed chain).